Gold assisted oxygen dissociation on a molybdenum-doped CaO(001) surface
Abstract
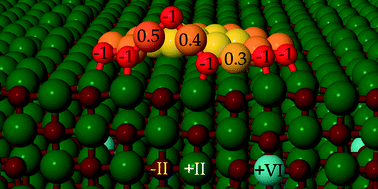
Using density functional theory (DFT) calculations, we address the adsorption of O2 and the coadsorption of gold species and oxygen molecules on a Mo-doped CaO(001) surface with 1.25% impurity concentration. With the help of the Born–Haber thermodynamic cycle, the enhanced binding of an oxygen molecule on Ca(Mo)O is attributed to energy gain owing to simultaneous electron transfer from the dopant to the molecule and lattice relaxations. We consider three coadsorption structures for an Au atom and O2 molecule with different Au–O2 distances. The calculations demonstrate that the coadsorption structures take one electron from the dopant and the O–Au–O chain structure is thermodynamically more stable than the molecular structure. The presence of multiple adsorbates introduces a competition for transferable electrons between adsorbates, which is seen in the variation of adsorption energies. Further calculations predict that the O–Au–O chain structures can form at the edge of a planar Au19, where the dissociative adsorption of an oxygen molecule is thermodynamically favoured nearly by 1 eV compared to the molecular adsorption and the dissociation barrier of ~0.6 eV. The dissociative adsorption remains energetically favored up to a full coverage of six molecules at the Au19 cluster edge with the formation of an oxidized zigzag Au–O–Au edge. The energetically favored dissociation is connected with the cluster's ability to donate charge to the anti-bonding states of the oxygen. Atomistic thermodynamics is applied to calculate the Gibbs free energy of oxygen adsorption. These calculations indicate that the oxidized Au cluster edge could be stable under ambient conditions of 1 atm pressure and 300 K temperature.
- This article is part of the themed collection: Nanocatalysis

 Please wait while we load your content...
Please wait while we load your content...