Iterative multitarget evolution dramatically enhances the enantioselectivity and catalytic efficiency of Bacillus subtilis esterase towards bulky benzoate esters of dl-menthol†
Abstract
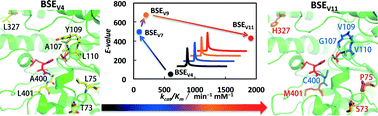
Structure-based directed evolution has been successfully applied to BSEV4, a variant of Bacillus subtilis esterase, for enantioselective hydrolysis of DL-menthyl esters with elevated thermostability. BSEV4 displayed only 90% enantiomeric excess (ee) in preparing L-menthol from DL-menthyl benzoate, whereas the quadruple mutant BSEV7, produced by iterative saturation mutagenesis, showed >99% ee. Partial deconvolution of BSEV7 by generating the four respective single mutants indicated that each of them only improved the enantioselectivity slightly, implying pronounced synergy when the mutations act together. BSEV9 and BSEV11, produced by site-directed mutagenesis and random mutagenesis, respectively, showed 2.5- and 20-fold higher activities than BSEV7 while retaining high E-values. With merely 0.1 g L−1 of BSEV11 loading (cell-free extract), 130 g L−1 of DL-menthyl benzoate was asymmetrically hydrolyzed within 6 h, resulting in an enantiopurity of >99% ee and a space-time yield of 138 g L−1 d−1. This suggests that it is feasible to establish an efficient bioprocess by simultaneously addressing multiple issues of biocatalyst performance using the iterative multi-target evolution approach.

 Please wait while we load your content...
Please wait while we load your content...