Octahedral Werner complexes with substituted ethylenediamine ligands: a stereochemical primer for a historic series of compounds now emerging as a modern family of catalysts†
Abstract
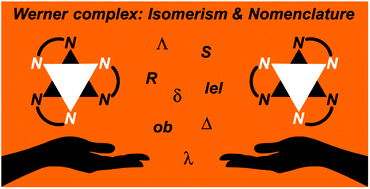
As reported by Alfred Werner in 1911–1912, salts of the formally D3 symmetric [Co(en)3]3+ (en = ethylenediamine) trication were among the first chiral inorganic compounds to be resolved into enantiomers, the absolute configurations of which are denoted Λ (left handed helix) or Δ (right handed helix). After a >100 year dormant period during which few useful reactions of these substitution inert complexes were described, carbon substituted derivatives have recently been found to be potent catalysts for enantioselective organic synthesis. This review systematically outlines the fascinating range of stereoisomers that can arise, such as conformers associated with the five membered chelate rings (λ/δ), alignment modes of the C–C bonds with the C3 symmetry axis (lel/ob), geometric isomers (fac/mer), and configurational diastereomers (R/S) arising from carbon stereocenters. These analyses demonstrate a profound stereochemical diversity that can be applied in catalyst optimization. Efforts are made to bridge the often orthogonal nomenclature systems inorganic and organic chemists employ to describe these phenomena.


 Please wait while we load your content...
Please wait while we load your content...