Kekulenes, cycloarenes, and heterocycloarenes: addressing electronic structure and aromaticity through experiments and calculations
Abstract
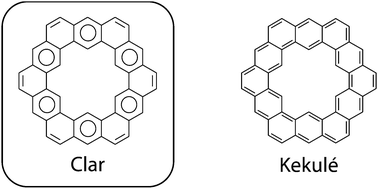
Kekulenes, cycloarenes, and heterocycloarenes have attracted much attention though the years, largely due to their electronic structure. The synthesis and characterization of these interesting molecules showed that their π electrons remained delocalized in individual benzenoid-type rings rather than globally delocalized in an annulenoid fashion. This discovery further suggested that the Clar bonding model, not the Kekulé model, is the best representation for depicting the bonding of large macrocyclic aromatic compounds. A plethora of computational studies suggest that cycloarenes gain little, if any, energetic stabilization from global delocalization, obviating the need for the concept of superaromaticity. More recently, cycloarenes have been suggested to serve as models for defects in graphene. Further study into this interesting set of compounds will continue to provide insights into fundamental questions about how aromatic compounds behave.
- This article is part of the themed collections: Primer and Challenges in Aromaticity: 150 Years after Kekulé’s Benzene

 Please wait while we load your content...
Please wait while we load your content...