Phase diagram and superconductivity of compressed zirconium hydrides†
Abstract
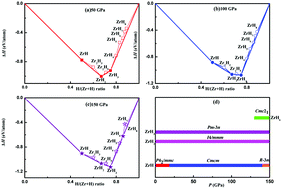
It is known that pressure can be applied to fundamentally alter the bonding patterns between the chemical elements. By employing an unbiased structure search method based on a particle swarm optimization (PSO) methodology, the phase diagram and crystal structures of Zr–H compounds are systematically investigated at a high pressure up to 150 GPa. Interestingly, some unexpectedly stable compounds with unusual chemical and physical properties are predicted to be formed, for example, four stable and metallic species with stoichiometries of ZrH, ZrH2, ZrH3, and ZrH6 are identified for the first time. It is interesting to note that Cmc21-ZrH6 adopts intriguing structures with H2 units. Surprisingly, it is found that Cmcm-ZrH is superconducting with Tc as high as 10.6 K. Our study opens a novel avenue for designing superconducting Zr–H compounds by applying pressure.


 Please wait while we load your content...
Please wait while we load your content...