A driving force for polypeptide and protein collapse†
Abstract
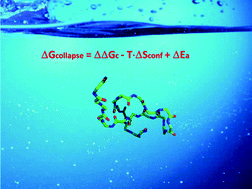
Experimental measurements and computational results have shown that polypeptide chains, made up of 15–25 glycine residues, collapse to compact structures in water at room temperature. This contrasts with the classic idea that the burial of nonpolar side chains, i.e., the hydrophobic effect, is the driving force of collapse and folding of polypeptides and proteins. It is thus necessary to find a different driving force for polyglycine collapse. The present study aims at showing that the hydrophobic effect has to be re-defined in terms of decrease in solvent-excluded volume associated with chain collapse so that it is characterized by a gain in translational entropy of water molecules. This indicates that the presence of nonpolar side chains is not so important for polypeptide and protein collapse, even though it may be fundamental for the attainment of a unique folded structure.


 Please wait while we load your content...
Please wait while we load your content...