Structural effects of soft nanoparticulate ligands on trace metal complexation thermodynamics†
Abstract
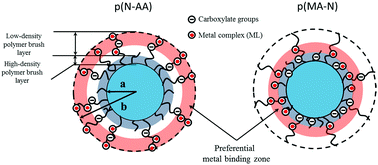
Metal binding to natural soft colloids is difficult to address due to the inherent heterogeneity of their reactive polyelectrolytic volume and the modifications of their shell structure following changes in e.g. solution pH, salinity or temperature. In this work, we investigate the impacts of temperature- and salinity-mediated modifications of the shell structure of polymeric ligand nanoparticles on the thermodynamics of divalent metal ions Cd(II)-complexation. The adopted particles consist of a glassy core decorated by a fine-tunable poly(N-isopropylacrylamide) anionic corona. According to synthesis, the charges originating from the metal binding carboxylic moieties supported by the corona chains are located preferentially either in the vicinity of the core or at the outer shell periphery (p(MA-N) and p(N-AA) particles, respectively). Stability constants (KML) of cadmium-nanoparticle complexes are measured under different temperature and salinity conditions using electroanalytical techniques. The obtained KML is clearly impacted by the location of the carboxylic functional groups within the shell as p(MA-N) leads to stronger nanoparticulate Cd complexes than p(N-AA). The dependence of KML on solution salinity for p(N-AA) is shown to be consistent with a binding of Cd to peripheral carboxylic groups driven by Coulombic interactions (Eigen–Fuoss mechanism for ions-pairing) or with particle electrostatic features operating at the edge of the shell Donnan volume. For p(MA-N) particulate ligands, a scenario where metal binding occurs within the intraparticulate Donnan phase correctly reproduces the experimental findings. Careful analysis of electroanalytical data further evidences that complexation of metal ions by core–shell particles significantly differ according to the location and distribution of the metal-binding sites throughout the reactive shell. This complexation heterogeneity is basically enhanced with increasing temperature i.e. upon significant increase of particle shell shrinking, which suggests that the contraction of the reactive phase volume of the particulate ligands promotes cooperative metal binding effects.


 Please wait while we load your content...
Please wait while we load your content...