Polyelectrolyte multilayer-cushioned fluid lipid bilayers: a parachute model†
Abstract
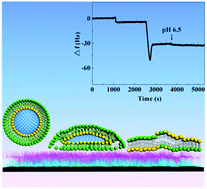
Lipid bilayer membranes supported on polyelectrolyte multilayers are widely used as a new biomembrane model that connects biological and artificial materials since these ultrathin polyelectrolyte supports may mimic the role of the extracellular matrix and cell skeleton in living systems. Polyelectrolyte multilayers were fabricated by a layer-by-layer self-assembly technique. A quartz crystal microbalance with dissipation was used in real time to monitor the interaction between phospholipids and polyelectrolytes in situ on a planar substrate. The surface properties of polyelectrolyte films were investigated by the measurement of contact angles and zeta potential. Phospholipid charge, buffer pH and substrate hydrophilicity were proved to be essential for vesicle adsorption, rupture, fusion and formation of continuous lipid bilayers on the polyelectrolyte multilayers. The results clearly demonstrated that only the mixture of phosphatidylcholine and phosphatidic acid (4 : 1) resulted in fluid bilayers on chitosan and alginate multilayers with chitosan as a top layer at pH 6.5. A coarse-grained molecular simulation study elucidated that the exact mechanism of the formation of fluid lipid bilayers resembles a “parachute” model. As the closest model to the real membrane, polyelectrolyte multilayer-cushioned fluid lipid bilayers can be appropriate candidates for application in biomedical fields.


 Please wait while we load your content...
Please wait while we load your content...