Second-order NLO responses of two-cavity inorganic electrides Lin@B20H26 (n = 1, 2): evolutions with increasing excess electron number and various B–B connection sites of B20H26†
Abstract
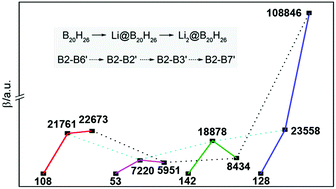
Confining excess electrons in a specific space is an effective strategy to design nonlinear optical (NLO) molecules. The complexants with excess electrons are usually organic compounds, but these compounds are thermally unstable and thus hardly meet the processing requirements of NLO materials. To obtain better thermostability and NLO response molecules, in this work, inorganic compounds of B20H26 isomers containing two cavities were proposed. With the two included cavities, B20H26 can be doped by one or two Li atoms to form electrides of Li@B20H26 and Li2@B20H26. These electrides show larger NLO responses, with respect to the corresponding undoped complexant of B20H26. Particularly, Li2@B20H26 has the largest β0 value of 108 846 a.u. (MP2/6-31+G(d) level) that is 850 times as large as that of corresponding B20H26. Moreover, the change of β0 values with excess electron number is remarkable for two of the isomers, and differences between the β0 values among those isomers are also significant owing to various B–B connection sites between the two cavities. Therefore, the present inorganic electrides have not only better performance due to the magnitude of their β0 values but also better behavior on the molecular-level modulation of NLO.


 Please wait while we load your content...
Please wait while we load your content...