Dynamic nitroxyl formation in the ammonia oxidation on platinum via Eley–Rideal reactions†
Abstract
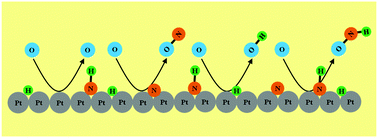
For over 90 years, nitroxyl (HNO) has been postulated to be an important reaction intermediate in the catalytic oxidation of ammonia to NO and its by-products (N2, N2O), but never proven to form or exist on catalytic surfaces. Here we show evidence from reactive ion beam experiments that HNO can form directly on the surface of polycrystalline Pt exposed to NH3via Eley–Rideal abstraction reactions of adsorbed NH by energetic O+ and O2+ projectiles. The dynamic formation of HNO in a single collision followed up by prompt rebound from the surface prevents subsequent reactive interactions with other surface adsorbates and enables its detection. In addition to HNO, NO and OH are also detected as direct products in what constitutes the concurrent abstraction of three surface adsorbates, namely NH, N, and H, by O+ projectiles with entirely predictable kinematics. While its relation to thermal catalysis may be tenuous, dynamic HNO formation could be important on grain surfaces of interstellar or cometary matter under astrophysical conditions.


 Please wait while we load your content...
Please wait while we load your content...