Unusual acid–base properties of the P4 molecule in hydrogen-, halogen-, and pnicogen-bonded complexes†
Abstract
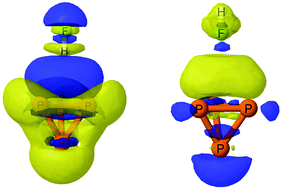
Ab initio MP2/aug′-cc-pVTZ calculations have been carried out to investigate hydrogen bonding, halogen bonding, and pnicogen bonding involving tetrahedral P4 and the FH, ClH, and FCl molecules. P4 has three unique interaction sites: at a vertex (designated the P1 atom); at an edge (the P2–P3 bond); and at the P2–P3–P4 face. The uniqueness of molecular P4 is its ability to act as an electron donor and an electron acceptor at the same site, except for the P2–P3 bond, which is only an electron donor. FCl and FH form five different complexes with P4, but ClH forms only three. The type of complex formed and its binding energy depend on both the interaction site of molecular P4 and the interacting molecule. For all complexes with FH, ClH, and FCl, the binding energies at a given site with the P4 molecule acting as the base are greater than the binding energies when P4 is the acid. Thus, P4 is a better electron donor than an electron acceptor. Charge-transfer interactions and EOM-CCSD spin–spin coupling constants across hydrogen, halogen, and pnicogen bonds are reported for all of the P4 complexes. Relative to 1J(Pi–Pj) in molecular P4, 1J(P1–P2) coupling constants decrease in absolute value and 1J(P2–P3) coupling constants increase in pnicogen-bonded complexes and the complex with FCl that has a P⋯F halogen bond. Absolute values of 1J(P1–P2) increase and those of 1J(P2–P3) decrease in hydrogen-bonded complexes and complexes with P⋯Cl halogen bonds. 1J(P1–P2) and 1J(P2–P3) exhibit a single linear correlation with the corresponding Pi–Pj distances.


 Please wait while we load your content...
Please wait while we load your content...