Lattice strain effects on doping, hydration and proton transport in scheelite-type electrolytes for solid oxide fuel cells†
Abstract
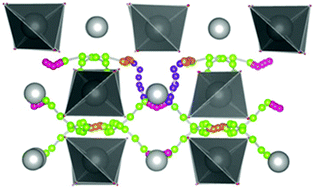
Lattice strain is considered a promising approach to modulate the structural and functional properties of oxide materials. In this study we investigate the effect of lattice strain on doping, hydration and proton transport for the family of scheelite-type proton conductors using both atomistic and DFT computational methods. The results suggest that tensile strain improves the dopant solubility and proton uptake of the material. The anisotropic proton pathways change from being within the a–b plane to being in the a–c plane. However, the predicted reduction in the migration barrier suggests that improvements in ionic conductivity due to lattice strain effects will be limited, in contrast with the work on oxide ion conduction. Such results are rationalized in terms of structural changes and differences in migration steps between oxide ions and protonic species.


 Please wait while we load your content...
Please wait while we load your content...