Electronic forces as descriptors of nucleophilic and electrophilic regioselectivity and stereoselectivity
Abstract
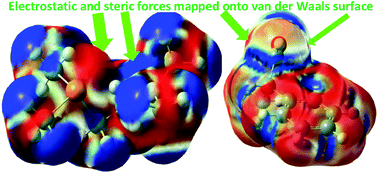
One of the main tasks of theoretical chemistry is to rationalize computational results with chemical insights. Key concepts of such nature include nucleophilicity, electrophilicity, regioselectivity, and stereoselectivity. While computational tools are available to predict barrier heights and other reactivity properties with acceptable accuracy, a conceptual framework to appreciate above quantities is still lacking. In this work, we introduce the electronic force as the fundamental driving force of chemical processes to understand and predict molecular reactivity. It has three components but only two are independent. These forces, electrostatic and steric, can be employed as reliable descriptors for nucleophilic and electrophilic regioselectivity and stereoselectivity. The advantages of using these forces to evaluate molecular reactivity are that electrophilic and nucleophilic attacks are featured by distinct characteristics in the electrostatic force and no knowledge of quantum effects included in the kinetic and exchange–correlation energies is required. Examples are provided to highlight the validity and general applicability of these reactivity descriptors. Possible applications in ambident reactivity, σ and π holes, frustrated Lewis pairs, and stereoselective reactions are also included in this work.


 Please wait while we load your content...
Please wait while we load your content...