Photochemical recovery of europium from non-aqueous solutions
Abstract
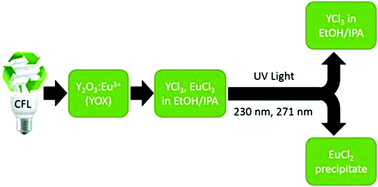
The photochemical recovery of europium from non-aqueous media, more specifically alcohols, is studied. The recovery was performed by photochemical reduction of europium(III) to europium(II) and subsequent removal as the insoluble EuCl2. Two charge transfer bands are present in the UV-C region, one originating from the alcohol (around 230 nm) and the other from the chloride anion (at 271 nm), which are responsible for the photochemical reduction when the solution is illuminated by a medium-pressure mercury lamp. When using different alcohol solvents, a trend is observed with regards to the removal rate and efficiency, following methanol (MeOH) < ethanol (EtOH) < isopropanol (IPA) <50/50 v/v ethanol/isopropanol (EtOH/IPA). This trend can be explained by the solubility of EuCl2 in the different solvents, and by the photon absorption at the wavelengths which provoke the reduction. In a 50/50 v/v EtOH/IPA solution, it is observed that addition of chloride ions (as LiCl) intensifies the chloride-to-europium(III) CT band, effectively increasing the photon absorption in the 260–340 nm wavelength region. Moreover, addition of extra chloride ions decreases the solubility of EuCl2, which in turn accounts for a better recovery efficiency. However, this beneficial effect disappears when the water content rises above 1.5 wt%. For an EtOH/IPA solution with a high chloride concentration and low water content, it is feasible to recover europium from binary europium/yttrium mixtures with an efficiency of up to 94.7% and a purity of 96.7–99.8%, depending on the Eu/Y molar ratio. For higher yttrium excess, the removal rate of europium is higher, which is explained by the ability of yttrium to coordinate water molecules, decreasing the free water content in the solution. The fact that a large excess of yttrium does not compromise the removal rate of europium from the solution, proves that this technique has potential for europium recovery from red lamp phosphors (Y2O3:Eu3+), which consist entirely of europium and yttrium with a Eu/Y molar ratio of 1/20–1/30.

 Please wait while we load your content...
Please wait while we load your content...