Tuning the vibrational coupling of H3O+ by changing its solvation environment†
Abstract
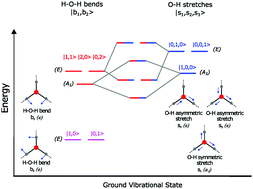
This study demonstrates how the intermode coupling in the hydronium ion (H3O+) is modulated by the composition of the first solvation shell. A series of rare gas solvated hydronium ions (H3O+Rg3, where Rg = Ne, Ar, Kr, and Xe) is examined via reduced-dimensional anharmonic vibrational (RDAV) ab initio calculations. We considered six key vibrational normal modes, namely: a hindered rotation, two H–O–H bends, and three O–H stretches. Between the O–H stretches and the H–O–H bends, the first is more sensitive to solvation strength. Our calculations revealed that the Fermi resonance between the first overtones of O–H bends and the fundamentals of O–H stretches led to complex spectral features from 3000 to 3500 cm−1. Such an interaction is not only sensitive to the type of rare gas messengers surrounding the H3O+ ion, it also exhibits an anomalous H → D isotope effect. Although it is accepted that visible combination tones (∼1900 cm−1) arise from the complex coupling between the hindered rotation and the H–O–H bends, the origin of their intensities is not yet clearly understood. We found that the intensity of these combination tones could be much stronger than their fundamental H–O–H bends. Within our theoretical framework, we tracked the combination tone's intensity back to the asymmetric O–H stretches. This simple notion of intensity borrowing is confirmed by examining eight complexes (H3O+·Rg3 and D3O+·Rg3) with spectral features awaiting experimental confirmations.


 Please wait while we load your content...
Please wait while we load your content...