A critical assessment of the mechanisms governing the formation of aqueous biphasic systems composed of protic ionic liquids and polyethylene glycol†
Abstract
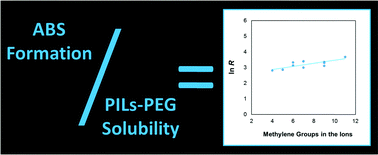
An extensive study on the formation of aqueous biphasic systems (ABS) using aqueous solutions of protic ionic liquids (PILs) and polyethylene glycol (PEG) was performed in order to understand the mechanisms underlying the phase separation. Aqueous solutions of PEG polymers with different molecular weights (600, 1000, 2000, and 3400 g mol−1) and several N-alkyl-, dialkyl-, and trialkyl-ammonium salts of acetate, propanoate, butanoate, hexanoate and octanoate were prepared and their ability to form ABS at several temperatures assessed. The ternary liquid–liquid phase diagrams were determined at several temperatures, as well as binary PIL (or salt)-PEG-1000 and salt-water solubility data to better clarify the mechanisms responsible for the phase separation. All data gathered indicate that the formation of PEG–PIL-based ABS is mainly governed by the PIL–PEG mutual interactions, where PILs with a higher solubility in the polymer exhibit a lower aptitude to form ABS displaying thus a smaller biphasic region, for which a direct correlation was identified. The effects of the molecular weight and temperature of the polymer were also addressed. The increase of the PEG hydrophobicity or molecular weight favours the phase separation, whereas the effect of temperature was found to be more complex and dependent on the nature of the PIL, with an increase or decrease of the biphasic regime with an increase in temperature.


 Please wait while we load your content...
Please wait while we load your content...