Study of entropic characteristics of strongly correlated systems using VO2 as a model case
Abstract
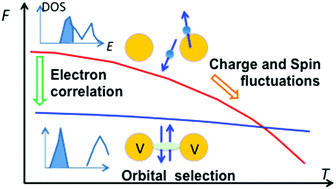
To explain the huge caloric effects often observed in the first-order electronic phase transition in the strongly correlated oxides, the entropic characteristics are investigated in VO2. By evaluating the spin and charge fluctuations based on the local moment model and the Sommerfeld coefficient in the high-temperature rutile phase, it is found that these fluctuations of the high-temperature phase are the main source of the entropic change during the transition. This mode of entropic change is realized by the quenching of these fluctuations owing to the formation of a singlet bonding state in the low-temperature monoclinic phase. By introducing oxygen deficiency, a vagueness in the gap at the Fermi level is confirmed by the transport data, the X-ray photoelectron spectra and also the electronic structure calculated by the first-principles calculations. In this case, the entropic feature at the transition is weakened. Consequently, the large caloric phenomena of the strongly correlated oxides are a result of the conversion of the internal energy gain owing to the orbital selection at the ground state into the free energy gain owing to the spin and charge fluctuations at finite temperature.

 Please wait while we load your content...
Please wait while we load your content...