A delicate case of unidirectional proton transfer from water to an aromatic heterocyclic anion
Abstract
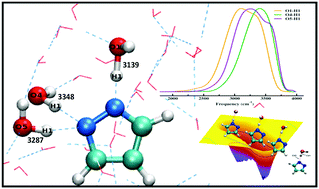
We present the characteristic proton transfer process from water to the pyrazole anion, infrared signatures of hydroxyl groups and the free energy profile of the process in aqueous solution combining first principles simulations, wavelet analysis and metadynamics. Our results show that the presence of minimum three water molecules in the gas phase cluster with a particular arrangement is sufficient to facilitate the proton transfer process from water to the anion. The overall reaction is very rapid in aqueous solution, and the free energy barrier for this process is found to be 4.2 kcal mol−1. One of the earlier reported fundamental reasons for the transfer of proton from water to the anion is the change in the acidity of OH groups surrounding the anion. We have correlated the stretching frequencies of the surrounding OH groups with this acidity. We find that the development of less energetic vibrational states, and the OH mode having lowest average stretching frequency contains the most acidic proton. A large frequency shift of the OH mode belonging to one of the surrounding water molecules is observed during the transfer of proton from water to the anion; this shift is due to the change in acidity of the adjacent hydroxyl groups in the vicinity of the anion.

 Please wait while we load your content...
Please wait while we load your content...