An unusual deprotonation trend in 2-(2′-hydroxyphenyl)pyridoimidazoles†
Abstract
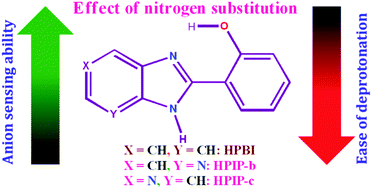
The anion sensitivity and the deprotonation nature of the nitrogenous analogues of 2-(2′-hydroxyphenyl)benzimidazole (HPBI) are investigated in a polar aprotic medium. It is observed that the substitution of pyridyl nitrogen enhances the anion sensitivity. However, despite the enhanced sensitivity of the nitrogenous analogues the deprotonation of these molecules in the presence of strong anions is less favored as compared to HPBI. This anomalous trend observed for the nitrogenous analogs is discussed and explained using theoretical calculations and experimental findings. It is also found that the sensitivity towards anions and the formation of anions also depend on the position of the pyridyl nitrogen.

 Please wait while we load your content...
Please wait while we load your content...