The segregation resistance of the Pt2ML/Os/Pd3Al sandwich catalyst for oxygen reduction reaction: a density functional theory study†
Abstract
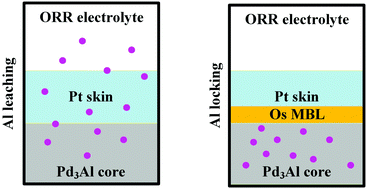
Pt1ML/Pd3Al, which comprises a Pd3Al core protected by a Pt monolayer, may experience Al dealloying because of the strong affinity of Al toward O. To circumvent this issue, the Pt2ML/Os/Pd3Al catalyst has been designed to suppress the migration of Al by inserting an Os monolayer at the interface between the Pd3Al core and two Pt monolayers. On the basis of segregation energies, Al leaching from the core to the 1st layer is determined to be endothermic even under O coverage, indicating an energetic preference for Al to reside in the core structure. The Pt2ML/Os/Pd3Al catalyst benefits from the energetic disadvantage of the inward movement of Os and the presence of the 2 ML Pt layer. As an ORR electrocatalyst, the relatively weak adsorption ability of Pt2ML/Os/Pd3Al suggests improved ORR activity. Finally, a representative OOH association mechanism with low reaction barriers of 0.46, 0.31, 0.38 and 0.41 eV for the OOH formation, OOH dissociation, OH formation and H2O formation steps suggests that the catalyst can effectively activate the O–O bond and eliminate OH, which can act as a catalytic poison. These findings suggest the design of stable sandwich catalysts as potential candidates for ORR electrocatalysis.

 Please wait while we load your content...
Please wait while we load your content...