Oxygen diffusion and surface exchange in the mixed conducting oxides SrTi1−yFeyO3−δ†
Abstract
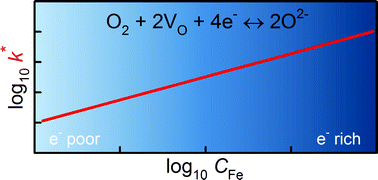
Oxygen transport in the mixed ionic–electronic conducting perovskite-oxides SrTi1−yFeyO3−δ (with y = 0.5 and y = 1.0) was studied by oxygen isotope exchange measurements. Experiments were performed on thin-film samples that were grown by Pulsed Laser Deposition (PLD) on MgO substrates. Isotope penetration profiles were introduced by 18O2/16O2 exchanges into the plane of the films at various temperatures in the range 773 < T/K < 973 at an oxygen activity aO2 = 0.5. Isotope profiles were determined subsequently by Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), and their analysis yielded tracer diffusion coefficients D* and oxygen surface exchange coefficients k*. Activation energies for oxygen diffusion ΔHD* and surface exchange ΔHk* were obtained. Isothermal values of D* and values of ΔHD* are compared with literature data as a function of Fe content. D* is seen to increase monotonically with Fe content; ΔHD* shows more complex behaviour. D* and ΔHD* are also compared with the predictions of defect-chemical models. Analogous comparisons with literature data for k* and ΔHk* indicate, in contrast to prior studies, no mechanistic difference between electron-poor and electron-rich materials. It is concluded that the single operative mechanism of surface exchange for the entire series of STF compositions requires conduction-band electrons (minority electronic charge-carriers).


 Please wait while we load your content...
Please wait while we load your content...