Tuning phase behaviour of PEG-functionalized ionic liquids from UCST to LCST in alcohol–water mixtures
Abstract
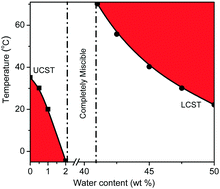
Thermo-responsive materials with reversible phase transition in molecular solvents are of great importance for catalysis reaction, product separation, and catalyst recycling among others. In this work, liquid–liquid phase transition of PEG-functionalized ionic liquids [PEGm(mim)2][NTf2]2 (m = 200, 400, 600, 800, 1000) in aliphatic alcohol (ethanol, 1-propanol or isopropanol) and in aqueous aliphatic alcohol was investigated by turbidity and dynamic light scattering (DLS) measurements, and the effects of the alkyl chain length of the alcohol molecules and molecular weight of the PEG middle block of the ionic liquids on the phase transition behaviour were examined. It was found that these ionic liquids exhibited a unique UCST phase transition in the aliphatic alcohol, but their liquid–liquid phase transition behaviour could be tuned precisely from UCST to LCST in aqueous aliphatic alcohol. Furthermore, temperature-dependent FT-IR and/or 1H NMR measurements were performed to understand the possible origin of phase behavior of both [PEGm(mim)2][NTf2]2 in the aliphatic alcohol and [PEG800(mim)2][NTf2]2 in ethanol-d6/H2O.


 Please wait while we load your content...
Please wait while we load your content...