Relation between oxygen stoichiometry and thermodynamic properties and the electronic structure of nonstoichiometric perovskite La0.6Sr0.4CoO3−δ†
Abstract
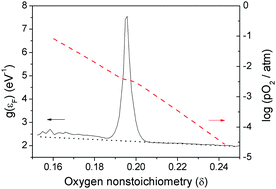
Continuous phase diagram 3 − δ − log pO2 − T of the nonstoichiometric perovskite La0.6Sr0.4CoO3−δ was obtained in a gas flow reactor by means of the quasi-equilibrium oxygen release technique. The thermodynamic properties of oxides were determined as a function of oxygen nonstoichiometry. Within the framework of the itinerant electron model, the dependence of the oxide nonstoichiometry on the oxygen activity was related to the density of electronic states near the Fermi level.


 Please wait while we load your content...
Please wait while we load your content...