Combined influence of ectoine and salt: spectroscopic and numerical evidence for compensating effects on aqueous solutions†
Abstract
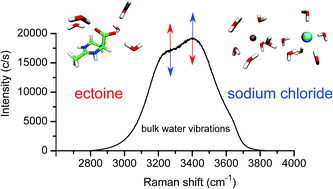
Ectoine is an important osmolyte, which allows microorganisms to survive in extreme environmental salinity. The hygroscopic effects of ectoine in pure water can be explained by a strong water binding behavior whereas a study on the effects of ectoine in salty solution is yet missing. We provide Raman spectroscopic evidence that the influence of ectoine and NaCl are opposing and completely independent of each other. The effect can be explained by the formation of strongly hydrogen-bonded water molecules around ectoine which compensate the influence of the salt on the water dynamics. The mechanism is corroborated by first principles calculations and broadens our understanding of zwitterionic osmolytes in aqueous solution. Our findings allow us to provide a possible explanation for the relatively high osmolyte concentrations in halotolerant bacteria.


 Please wait while we load your content...
Please wait while we load your content...