Conformation-controlled hydrogen storage in the CAU-1 metal–organic framework†
Abstract
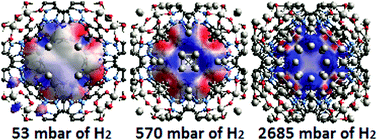
We have studied the mechanism of hydrogen storage in the aluminium based metal–organic framework CAU-1 or [Al4(OH)2(OCH3)4(O2C-C6H3NH2-CO2)3] using a complementary multidisciplinary approach of volumetric gas sorption analysis, in situ neutron diffraction and spectroscopy and ab initio calculations. The structure of CAU-1 forms two different types of microporous cages: (i) an octahedral cage with a diameter of about 10 Å and (ii) a tetrahedral cage with a diameter of about 5 Å. Though all metal sites of CAU-1 are fully coordinated, the material exhibits relatively high storage capacities, reaching 4 wt% at a temperature of 70 K. Our results reveal that hydrogen sorption is dominantly driven by cooperative guest–guest interactions and interactions between guest hydrogen molecules and organic linkers. The adsorption of hydrogen on the organic linkers leads to the contraction of the host framework structure and as a result to changes in the electronic potential surface inside the pores. This, in turn, leads to cooperative rearrangement of the molecules inside the pores and to the formation of additionally occupied positions, increasing hydrogen uptake. At the final stage we observe the formation of solid amorphous hydrogen inside the pores.


 Please wait while we load your content...
Please wait while we load your content...