CO tolerance of Pt/FeOx catalyst in both thermal catalytic H2 oxidation and electrochemical CO oxidation: the effect of Pt deficit electron state†
Abstract
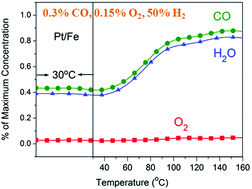
CO poisoning of Pt catalysts is one of the major challenges to the commercialization of proton exchange membrane fuel cells. One promising solution is to develop CO-tolerant Pt-based catalysts. A facilely synthesized Pt/FeOx catalyst exhibited outstanding CO tolerance in the oxidation of H2 and electrochemical CO stripping. Light-off temperature of H2O formation over Pt/FeOx was achieved even below 30 °C in the presence of 3000 ppm CO at a space velocity of 18 000 mL g−1cat h−1. For the electrochemical oxidation of CO, the onset and peak potentials decreased by 0.17 V and 0.10 V, respectively, in comparison with those of commercial Pt/C. More importantly, by a combination of hard X-ray photoemission spectroscopy and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) studies it was found that the decreased electron density of Pt in Pt/FeOx enhanced the mobility of adsorbed CO, suppressed Pt–CO bonding and significantly increased the CO tolerance of Pt/FeOx.


 Please wait while we load your content...
Please wait while we load your content...