Contrasting effects of pH on the modulation of the structural integrity of hemoglobin induced by sodium deoxycholate†
Abstract
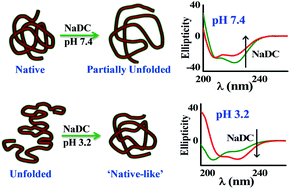
Bile salt-mediated conformational modification of hemoglobin (Hb) was examined at three different pHs i.e., 3.2, 7.4 and 9.0. The added bile salt, sodium deoxycholate (NaDC), decreases the α-helicity in Hb (α-helix: 71.3% → 61.7% in the presence of 9.6 mM NaDC, and 83.2% → 66.2% in the presence of 14 mM NaDC, at pH 7.4 and 9.0, respectively), while a reverse pattern of modification in the Circular Dichroism (CD) spectra of Hb is found at pH 3.2. The acid-induced denatured Hb (pH 3.2) regains its structural integrity by changing conformation from a random coil to an α-helix rich secondary structure upon addition of NaDC (α-helix: 10.4% → 53.4%, β-sheet: 31.0% → 18.5% and random coil: 58.6% → 28.1%, in the presence of 0.65 mM NaDC). Also, a step-wise binding interaction pattern of Hb with NaDC was revealed at pH 7.4 and 9.0 upon variation of steady-state fluorescence intensity and average lifetime of Hb. From the fluorescence lifetime decay pattern, the decrement of energy transfer from Trp to a heme group was found upon the addition of NaDC at pH 7.4 and 9.0. However, at pH 3.2, the modification of the time-resolved fluorescence decay behavior of Hb within NaDC is typically reversed, where the energy transfer from Trp to heme is restored to some extent. Thermodynamic analysis suggests that the Hb–NaDC binding interaction is characterized by a dominant entropic contribution interpreted on the basis of release of ordered water molecules to the bulk aqueous phase.

 Please wait while we load your content...
Please wait while we load your content...