Enhanced hydrogen desorption properties of LiAlH4 by doping lithium metatitanate
Abstract
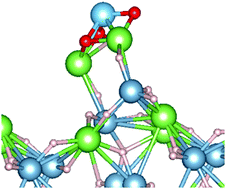
High efficiency catalysts are needed to improve the kinetics of complex hydrides for practical applications. In this study, lithium metatitanate (Li2TiO3) is introduced in lithium alanate (LiAlH4), and the catalytic effect for notable complex/metal hydrides, such as LiAlH4, is investigated. Experiment results indicate that Li2TiO3 improves the kinetics of LiAlH4. In particular, Li2TiO3 dramatically improves the onset temperature of LiAlH4, which decreases to 75 °C and is within the temperature range for use in proton exchange membrane fuel cells. Transmission electron microscopy (TEM) observations help understand the catalytic effect of Li2TiO3 in the nanoscale. First principles calculations also show the improvement of H− and Li+ mobility by doping Li2TiO3, where calculations indicate that the physical origin of the catalytic effect is due to two factors: charge transfer and minor surface relaxation. Thus, experimental and theoretical evidence reveals the catalytic mechanism of Li2TiO3 in LiAlH4.


 Please wait while we load your content...
Please wait while we load your content...