Electrostatics-mediated α-chymotrypsin inhibition by functionalized single-walled carbon nanotubes†
Abstract
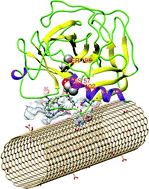
The α-chymotrypsin (α-ChT) enzyme is extensively used for studying nanomaterial-induced enzymatic activity inhibition. A recent experimental study reported that carboxylized carbon nanotubes (CNTs) played an important role in regulating the α-ChT activity. In this study, parallel tempering Monte Carlo and molecular dynamics simulations were combined to elucidate the interactions between α-ChT and CNTs in relation to the CNT functional group density. The simulation results indicate that the adsorption and the driving force of α-ChT on different CNTs are contingent on the carboxyl density. Meanwhile, minor secondary structural changes are observed in adsorption processes. It is revealed that α-ChT interacts with pristine CNTs through hydrophobic forces and exhibits a non-competitive characteristic with the active site facing towards the solution; while it binds to carboxylized CNTs with the active pocket through a dominant electrostatic association, which causes enzymatic activity inhibition in a competitive-like mode. These findings are in line with experimental results, and well interpret the activity inhibition of α-ChT at the molecular level. Moreover, this study would shed light on the detailed mechanism of specific recognition and regulation of α-ChT by other functionalized nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...