Suppressing the dissolution of polysulfides with cosolvent fluorinated diether towards high-performance lithium sulfur batteries†
Abstract
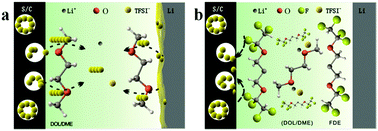
The dissolution and shuttle of polysulfides in electrolytes cause severe anode corrosion, low coulombic efficiency, and a rapid fading of the capacity of lithium–sulfur batteries. Fluorinated diether (FDE) was selected as a cosolvent in traditional ether electrolytes to suppress the dissolution of polysulfides. The modified electrolytes lead to a negligible solubility of polysulfides, as well as decreased corrosion of the lithium anode. In an optimal system, the cells show improved cycling performance with an average coulombic efficiency of above 99% and a highly stable reversible discharge capacity of 701 mA h g−1 after 200 cycles at a 0.5C rate. A combination of electrochemical studies and X-ray photoelectron spectroscopy demonstrates the sulfur reduction mechanism with three voltage plateaus.

 Please wait while we load your content...
Please wait while we load your content...