Accurate thermodynamic properties of gas phase hydrogen bonded complexes†
Abstract
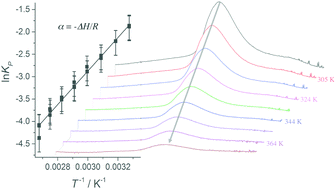
We have measured the infrared spectra of ethanol·dimethylamine and methanol·dimethylamine complexes in the 299–374 K temperature range, and have determined the enthalpy of complex formation (ΔH) to be −31.1 ± 2 and −29.5 ± 2 kJ mol−1, respectively. The corresponding values of the Gibbs free energy (ΔG) are determined from the experimental integrated absorbance and a calculated oscillator strength of the OH-stretching vibrational transition to be 4.1 ± 0.3 and 3.9 ± 0.3 kJ mol−1 at 302 and 300 K, respectively. The entropy, ΔS is determined from the values of ΔH and ΔG to be −117 ± 7 and −111 ± 10 J (mol K)−1 for the ethanol·dimethylamine and methanol·dimethylamine complexes, respectively. The determined ΔH, ΔG and ΔS values of the two complexes are similar, as expected by the similarity to their donor molecules ethanol and methanol. Values of ΔH, ΔG and ΔS in chemical reactions are often obtained from quantum chemical calculations. However, these calculated values have limited accuracy and large variations are found using different methods. The accuracy of the present ΔH, ΔG and ΔS values is such that the benchmarking of theoretical methods is possible.


 Please wait while we load your content...
Please wait while we load your content...