Effects of pore size and surface charge on Na ion storage in carbon nanopores
Abstract
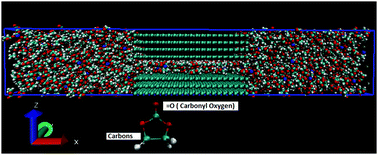
Na ion batteries (NIBs) are considered as a promising low cost and sustainable energy storage technology. To better design nanoporous carbons as anode materials for NIBs, molecular dynamics simulations have been employed to study the behavior of Na+ ions (as well as PF6− ions) confined within carbon nanopores, in the presence of non-aqueous (organic) solvent. The effects of pore size and surface charge density were quantified by calculating ionic density profiles and concentration within the pores. Carbon slit pores of widths 0.72–10 nm were considered. The carbon surfaces were charged with densities of 0 (neutral pores), −0.8e nm−2, −1.2e nm−2, and −2e nm−2. Organic solutions of Na+ and PF6− at 1 M concentrations were considered under operating conditions of sodium ion batteries. As the surface charge density increases, more Na+ ions enter the pores. In all pores, when the surface is highly charged the Na+ ions move toward the negatively charged graphene surfaces because of counterion condensation effects. In some instances, our results reveal the formation of multiple layers of adsorbed Na+ inside the pores. Both the nanopore width and surface charge alter the density profiles of ions and solvent inside the pores.

 Please wait while we load your content...
Please wait while we load your content...