Calcium-decorated carbon nanostructures for the selective capture of carbon dioxide
Abstract
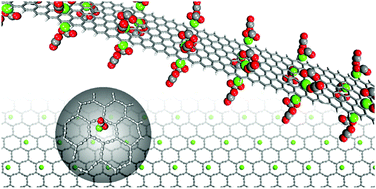
The development of advanced materials for CO2 capture is of great importance for mitigating climate change. In this paper, we outline our discovery that calcium-decorated carbon nanostructures, i.e., zigzag graphene nanoribbons (ZGNRs), carbyne, and graphyne, have great potential for selective CO2 capture, as demonstrated via first-principles calculations. Our findings show that Ca-decorated ZGNRs can bind up to three CO2 molecules at each Ca atom site with an adsorption energy of ∼−0.8 eV per CO2, making them suitable for reversible CO2 capture. They adsorb CO2 molecules preferentially, compared with other gas molecules such as H2, N2, and CH4. Moreover, based on equilibrium thermodynamical simulations, we confirm that Ca-decorated ZGNRs can capture CO2 selectively from a gas mixture with a capacity of ∼4.5 mmol g−1 under ambient conditions. Similar results have been found in other carbon nanomaterials, indicating the generality of carbon based nanostructures for selective CO2 capture under ambient conditions.

 Please wait while we load your content...
Please wait while we load your content...