Reactivity of electrophilic chlorine atoms due to σ-holes: a mechanistic assessment of the chemical reduction of a trichloromethyl group by sulfur nucleophiles†
Abstract
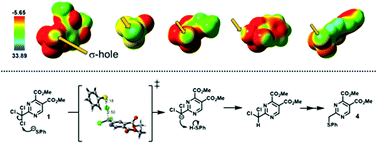
σ-Holes are shown to promote the electrophilic behavior of chlorine atoms in a trichloromethyl group when bound to an electron-withdrawing moiety. A halogen bond-type non-covalent interaction between a chlorine atom and a negatively charged sulfur atom takes place, causing the abstraction of such a chlorine atom while leaving a carbanion, subsequently driving the chemical reduction of the trichloromethyl group to a sulfide in a stepwise process. The mechanism for the model reaction of trichloromethyl pyrimidine 1 with thiophenolate and thiophenol to yield phenylsulfide 4 was followed through 1H-NMR and studied using DFT transition state calculations, and the energy profile for this transformation is fully discussed. MP2 calculations of the electrostatic potential were performed for a series of trichloromethyl compounds in order to assess the presence of σ-holes and quantify them by means of the maximum surface electrostatic potential. Such calculations showed that the chlorine atoms behave as electrophilic leaving groups toward a nucleophilic attack, opening a new possibility in the synthetic chemistry of the trichloromethyl group.


 Please wait while we load your content...
Please wait while we load your content...