Electronic structure and energy decomposition analyses as a tool to interpret the redox potential ranking of naphtho-, biphenyl- and biphenylene-quinone isomers
Abstract
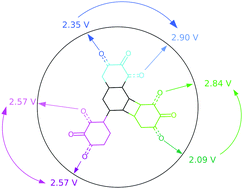
By calling on modelling approaches we have performed a comparative study on the redox properties of various naphtho-, biphenyl- and biphenylene-quinone isomers. These different compounds exhibit as a whole a redox potential range between 2.09 and 2.90 V vs. Li+/Li. A specific methodology was used to decrypt the interplay among isomerism, aromaticity and antiaromaticity modifications and the stabilization/destabilization effects due to other molecular components on this key electrochemical feature for electrode materials of batteries. In particular, energy decomposition analysis, within the Quantum Theory of Atoms in Molecules, along with the electron and electron spin population changes upon reduction nicely rationalise the observed potential trends. While 1,2- and 2,3-isomers show the highest/lowest redox potential in the biphenylene-quinone series, a reverse trend is observed for the naphtho-quinone, the compound having the two carbonyl groups on distinct rings being characterized by an intermediate value in both cases. There is instead almost no differentiation between 1,2 and 2,3 isomers for the biphenyl-quinone family.

 Please wait while we load your content...
Please wait while we load your content...