Shuttle inhibition by chemical adsorption of lithium polysulfides in B and N co-doped graphene for Li–S batteries
Abstract
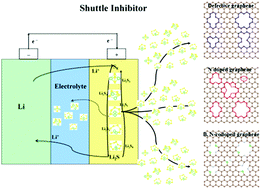
The advance of lithium sulfur batteries is now greatly restricted by the fast capacity fading induced by shuttle effect. Using first-principles calculations, various vacancies, N doping, and B,N co-doping in graphene sheets have been systematically explored for lithium polysufides entrapped in Li–S batteries. The Li⋯S, Li⋯C, Li⋯N and S⋯B bonds and Hirshfeld charges in the Li2S6 adsorbed defective graphene systems have been analyzed to understand the intrinsic mechanism of retaining lithium polysulfides in these systems. Total and local densities of states analyses elucidate the strongest adsorption sites among the N and B–N co-doped graphene systems. The overall electrochemical performance of Li–S batteries varies with the types of defects in graphene. Among the defective graphene systems, only the reconstructed pyrrole-like vacancy is effective for retaining lithium polysulfides. N doping induces a strong Li⋯N interaction in the defective graphene systems, in which the pyrrolic N rather than the pyridinic N plays a dominant role in trapping of lithium polysulfides. The shuttle effect can be further depressed via pyrrolic B,N co-doped defective graphene materials, especially the G–B–N-hex system with extremely strong adsorption of lithium polysulfides (4–5 eV), and simultaneous contribution from the strong Li⋯N and S⋯B interactions.

 Please wait while we load your content...
Please wait while we load your content...