Photofragmentation mechanisms in protonated chiral cinchona alkaloids†
Abstract
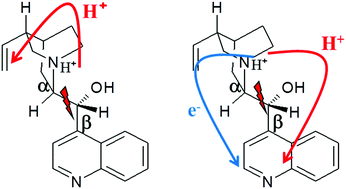
The photo-stability of protonated cinchona alkaloids is studied in the gas phase by a multi-technique approach. A multi-coincidence technique is used to demonstrate that the dissociation is a direct process. Two dissociation channels are observed. They result from the C8–C9 cleavage, accompanied or not by hydrogen migration. The branching ratio between the two photo-fragments is different for the two pseudo-enantiomers quinine and quinidine. Mass spectrometry experiments coupling UV photo-dissociation of the reactants and structural characterization of the ionic photo-products by Infra-Red Multiple Photo-Dissociation (IRMPD) spectroscopy provide unambiguous information on their structure. In addition, quantum chemical calculations allow proposing a reactive scheme and discussing it in terms of the ground-state geometry of the reactant.


 Please wait while we load your content...
Please wait while we load your content...