Kinetics of self-assembled monolayer formation on individual nanoparticles†
Abstract
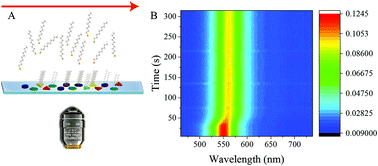
Self-assembled monolayer (SAM) formation of alkanethiols on nanoparticle surfaces is an extensively studied surface reaction. But the nanoscale aspects of the rich microscopic kinetics of this reaction may remain hidden due to ensemble-averaging in colloidal samples, which is why we investigated in real-time how alkanethiol SAMs form on a single Ag nanoparticle. From single-nanoparticle trajectories obtained using in situ optical spectroscopy, the kinetics of SAM formation appears to be limited by the growth of the layer across the nanoparticle surface. A significant spread in the growth kinetics is seen between nanoparticles. The single-nanoparticle rate distributions suggest two distinct modes for SAM growth: spillover of adsorbed thiols from the initial binding sites on the nanoparticle and direct adsorption of thiol from solution. At low concentrations, wherein direct adsorption from solution is not prevalent and growth takes place primarily by adsorbate migration, the SAM formation rate was less variable from one nanoparticle to another. On the other hand, at higher thiol concentrations, when both modes of growth were operative, the population of nanoparticles with inherent variations in surface conditions and/or morphology exhibited a heterogeneous distribution of rates. These new insights into the complex dynamics of SAM formation may inform synthetic strategies for ligand passivation and functionalization of nanoparticles and models of reactive adsorption and catalysis on nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...