Feynman force components: basis for a solution to the covalent vs. ionic dilemma†
Abstract
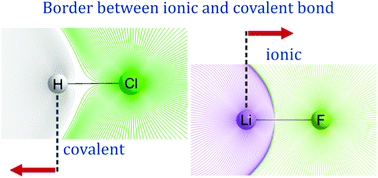
The Hellmann–Feynman theorem, when applied to nuclear coordinates in a molecular system, states that Feynman forces, i.e. forces acting on a nucleus in a molecule, are solely of an electrostatic nature. This theorem is described by Slater as “the most powerful” theorem applicable to molecules. However, its possibilities have hardly been harnessed. This work presents the use of the Hellmann–Feynman theorem in conjunction with the partitioning of the molecular space into atoms in the spirit of the quantum theory of atoms in molecules (QTAIM). Homopolar and heteropolar diatomic molecules of varying polarity are studied in the context of Feynman force components, i.e. the components exerted on each nucleus by the other nucleus and by the electron density distributions of each of the atoms. These results are further related to electronegativity differences used in the differentiation between covalent and ionic bond. The approach based on the directions of Feynman force components gives physical fundamentals for covalent vs. ionic bond distinction without referring to the electronegativity concept.


 Please wait while we load your content...
Please wait while we load your content...