Transferability of a coarse-grained atactic polystyrene model: the non-bonded potential effect†
Abstract
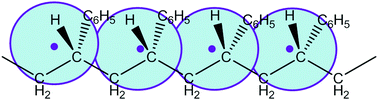
In this paper, we construct an efficient and simple coarse grained (CG) model for atactic polystyrene (PS) by using a 1 : 1 mapping scheme at 463 K and 1 atm pressure and derive the corresponding bonded and non-bonded potentials in the CG force field (FF) via a direct Boltzmann inversion approach and a combined structure-based and thermodynamic quantities-based CG method, respectively. For computational considerations, the non-bonded interaction between CG particles is described by Lennard-Jones (LJ) type potentials, and both the radial distribution function (RDF) and the bulk density of the atomistic simulations are taken as target properties in the parameterization of the two LJ parameters. To shed light on the choice of LJ forms of CG non-bonded potentials when designing the CG models, a series of CG models with different LJ potentials are constructed and compared in order to understand how the quality of a CG model in reproducing the structure and thermodynamic properties of chemically realistic systems is affected by the choice of non-bonded potentials. We find that with our structural and thermodynamics combined CG method to construct the CG FF at a single thermodynamic state point without any temperature dependent LJ potential correction and/or pressure optimization, the resulting CG models possess good temperature transferability in a wide range of temperatures 300–600 K, where both the target properties and several other static properties (such as thermal expansion coefficient and mean-square radius of gyration) are generally reproduced. Furthermore, the non-bonded LJ potential influences the density response of CG models to the temperature change, i.e., CG models with harder LJ potentials show better temperature transferability than the softer ones. Meanwhile, the derived Tg increases with increasing LJ repulsion strength while thermal expansion coefficients in both melt and glass states are lowered as the LJ potential hardens. With regard to the local conformation and local packing distribution functions, varying non-bonded LJ potential hardness influences only the magnitude of the peak height but does not affect the peak position, in particular the magnitude of the non-bonded potential effect on local distribution functions becomes stronger at lower temperatures. More specifically, this effect on the local chain conformation statistics at the CG level is different for the distribution of bond-lengths, bond angles and dihedrals. As a result, the size of the CG chains is fairly insensitive to the non-bonded LJ potentials within 300–600 K. In short, the CG model with the harder LJ-type non-bonded CG potential is a more realistic representation of excluded volume interactions of the underlying atomistic PS monomer and thus has the potential to generate a higher Tg to match with the atomistic systems.

 Please wait while we load your content...
Please wait while we load your content...