A chemical chaperone induces inhomogeneous conformational changes in flexible proteins†
Abstract
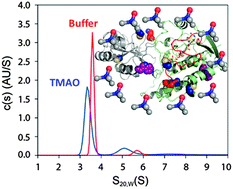
Organic osmolytes also known as chemical chaperones are major cellular compounds that favor, by an unclear mechanism, protein's compaction and stabilization of the native state. Here, we have examined the chaperone effect of the naturally occurring trimethylamine N-oxide (TMAO) osmolyte on a loosely packed protein (LPP), known to be a highly flexible form, using an apoprotein mutant of the flavin-dependent RNA methyltransferase as a model. Thermal and chemical denaturation experiments showed that TMAO stabilizes the structural integrity of the apoprotein dramatically. The denaturation reaction is irreversible indicating that the stability of the apoprotein is under kinetic control. This result implies that the stabilization is due to a TMAO-induced reconfiguration of the flexible LPP state, which leads to conformational limitations of the apoprotein likely driven by favorable entropic contribution. Evidence for the conformational perturbation of the apoprotein had been obtained through several biophysical approaches notably analytical ultracentrifugation, circular dichroism, fluorescence spectroscopy, labelling experiments and proteolysis coupled to mass spectrometry. Unexpectedly, TMAO promotes an overall elongation or asymmetrical changes of the hydrodynamic shape of the apoprotein without alteration of the secondary structure. The modulation of the hydrodynamic properties of the protein is associated with diverse inhomogenous conformational changes: loss of the solvent accessible cavities resulting in a dried protein matrix; some side-chain residues initially buried become solvent exposed while some others become hidden. Consequently, the TMAO-induced protein state exhibits impaired capability in the flavin binding process. Our study suggests that the nature of protein conformational changes induced by the chemical chaperones may be specific to protein packing and plasticity. This could be an efficient mechanism by which the cell controls and finely tunes the conformation of the marginally stable LPPs to avoid their inappropriate protein/protein interactions and aggregation.

 Please wait while we load your content...
Please wait while we load your content...