Hierarchical spatial heterogeneity in liquid crystals composed of graphene oxides†
Abstract
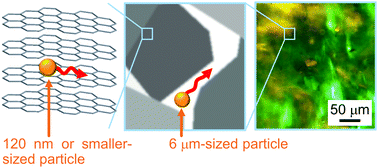
Graphene oxide (GO) is a class of two-dimensional materials with a thickness of about 1 nm and a broad distribution of lateral dimension commonly approaching several micrometers. A dispersion of GOs in water often forms a liquid crystal, which is expected to be a promising precursor for the fabrication of carbon-based materials with well-ordered structures. To accelerate the application of GO-based liquid crystals, their structures and physical properties at various sizes must be well understood. To that end, we examined the local rheological properties of GO-based liquid crystals in the nematic phase using a particle tracking technique, where local properties can be accessed by observing the thermal motion of embedded probe particles. Particle diffusion was spatially heterogeneous, and depended on the size of the particles. Such a size-dependent heterogeneity can be associated with a hierarchical local environment, which is time-dependent for this system. The anisotropic particle diffusion originated from particles trapped in between the GO layers and in isotropic-like regions. The aggregation states of the GO dispersion composed of nematic and isotropic-like regions were observed using confocal laser scanning microscopy.

 Please wait while we load your content...
Please wait while we load your content...