Effects of extending the π-conjugation of the acetylide ligand on the photophysics and reverse saturable absorption of Pt(ii) bipyridine bisacetylide complexes†
Abstract
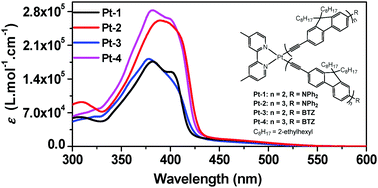
The synthesis and photophysics of four platinum(II) bipyridine (bpy) bisacetylide complexes with different degrees of π-conjugation and an electron-donating diphenylamino (NPh2) or electron-withdrawing benzothiazolyl (BTZ) terminal substituent on the acetylide ligands are reported. The UV-vis absorption spectra of these complexes are composed of intense ligand-localized 1π,π* transitions at 330–430 nm and broad, moderately strong ligand-to-ligand charge transfer/metal-to-ligand charge transfer (1LLCT/1MLCT) transitions at 430–530 nm. All complexes are phosphorescent in solutions at room temperature and exhibit very broad and moderately strong triplet excited-state absorption in the visible to the NIR spectral region (425–800 nm). It is found that extending the π-conjugation of the acetylide ligands via adding one or two more ethynylfluorenyl unit(s) to the acetylide ligand does not change the energies of the 1π,π* and 1LLCT/1MLCT transitions pronouncedly except for increasing the molar extinction coefficients of the 1π,π* transitions. The emitting triplet excited states of the four complexes are the 3MLCT/3LLCT states and have the same energy. However, the complex that contains the tris(ethynylfluorenyl) units and the terminal NPh2 substituent on the acetylide ligand exhibits longer triplet lifetimes than the corresponding complex that has the bis(ethynylfluorenyl) units. The transient absorption band maxima of the complexes with tris(ethynylfluorenyl) units are slightly red-shifted in comparison to those of their respective counterparts with bis(ethynylfluorenyl) units. The nature of the terminal substituent does not influence the parentage and energies of the lowest singlet and triplet excited states. However, the triplet excited-state lifetimes of the complexes with the NPh2 terminal substituent on the bis(ethynylfluorenyl) or tris(ethynylfluorenyl) ligands are much longer than that of their counterpart with monofluorenylacetylide ligands; while the triplet lifetimes of the complexes containing the BTZ terminal substituent are similar to their counterpart with monofluorenylacetylide ligands. All complexes exhibit strong reverse saturable absorption (RSA) at 532 nm for nanosecond laser pulses.

 Please wait while we load your content...
Please wait while we load your content...