Troponin structure: its modulation by Ca2+ and phosphorylation studied by molecular dynamics simulations†
Abstract
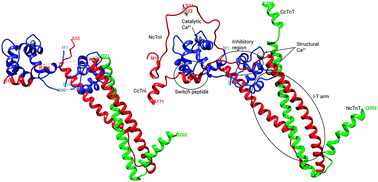
The only available crystal structure of the human cardiac troponin molecule (cTn) in the Ca2+ activated state does not include crucial segments, including the N-terminus of the cTn inhibitory subunit (cTnI). We have applied all-atom molecular dynamics (MD) simulations to study the structure and dynamics of cTn, both in the unphosphorylated and bis-phosphorylated states at Ser23/Ser24 of cTnI. We performed multiple microsecond MD simulations of wild type (WT) cTn (6, 5 μs) and bisphosphorylated (SP23/SP24) cTn (9 μs) on a 419 amino acid cTn model containing human sequence cTnC (1–161), cTnI (1–171) and cTnT (212–298), including residues not present in the crystal structure. We have compared our results to previous computational studies, and proven that longer simulations and a water box of at least 25 Å are needed to sample the interesting conformational shifts both in the native and bis-phosphorylated states. As a consequence of the introduction into the model of the C-terminus of cTnT that was missing in previous studies, cTnC–cTnI interactions that are responsible for the cTn dynamics are altered. We have also shown that phosphorylation does not increase cTn fluctuations, and its effects on the protein–protein interaction profiles cannot be assessed in a significant way. Finally, we propose that phosphorylation could provoke a loss of Ca2+ by stabilizing out-of-coordination distances of the cTnC's EF hand II residues, and in particular Ser 69.

 Please wait while we load your content...
Please wait while we load your content...