Formation of a thermally stable bilayer of coadsorbed intact and deprotonated thymine exploiting the surface corrugation of rutile TiO2(110)†
Abstract
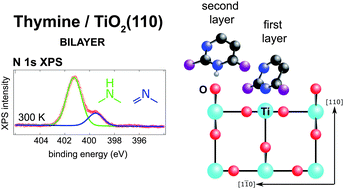
The adsorption of thymine, a pyrimidine based nucleobase, was studied on the (110) termination of rutile titanium dioxide in order to understand the thermal stability and gross structural parameters of the interaction between a strongly polar adsorbate and a highly corrugated transition metal oxide surface. Near-edge X-ray absorption fine structure (NEXAFS), X-ray photoelectron spectroscopy (XPS), temperature programmed XPS and temperature programmed desorption indicated the growth of a room temperature stable bilayer, which could only be removed by annealing to 450 K. The remaining first layer was remarkably robust, surviving annealing up to 550 K before undergoing N–H bond scission. The comparison to XPS of a sub-monolayer exposure of 1-methyluracil shows that the origin of the room temperature stable bilayer is not intermolecular interactions. This discovery, alongside the deprotonation of one of the first layer's pyrimidinic nitrogen atoms at room temperature, suggests that the thymine molecules in the first layer bind to the undercoordinated surface Ti atoms, and the second layer thymine molecules coordinate with the bridging oxygen atoms which protrude above the Ti surface plane on the (110) surface. The NEXAFS results indicate an almost upright orientation of the molecules in both layers, with a 30 ± 10° tilt away from the surface normal.

 Please wait while we load your content...
Please wait while we load your content...