Ice nucleation of an insect lipoprotein ice nucleator (LPIN) correlates with retardation of the hydrogen bond dynamics at the myo-inositol ring
Abstract
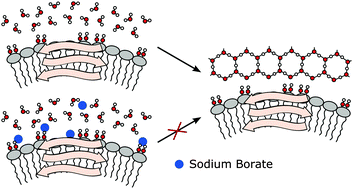
Remarkably little is known about the mechanism of action of ice nucleation proteins (INPs), although their ability to trigger ice nucleation could be used in a broad variety of applications. We present CD measurements of an insect lipoprotein ice nucleator (LPIN) which show that the lipoproteins consist of a high amount of β-structures (35%). Terahertz absorption spectroscopy is used to probe the influence of the LPIN on the H-bond network dynamics. We observe a small, but significant THz excess, as an indication of an influence on the H-bond network dynamics. When adding the ice nucleation inhibitor sodium borate, this effect is considerably reduced, similar to that observed before for antifreeze glycoproteins (AFGPs). We propose that myo-inositol, the functional group of phosphatidylinositols, is crucial for the observed change of the H-bond network dynamics of hydration water. This hypothesis is confirmed by additional THz experiments which revealed that the influence of myo-inositol on the hydrogen bond network can be blocked by sodium borate, similar to the case of LPINs. Interestingly, we find a less significant effect when myo-inositol is replaced for chiro- and allo-inositol which underlines the importance of the exact positioning of the OH groups for the interaction with the H-bond network. We propose that a local ordering of water molecules is supporting ice nucleation activity for the LPIN in a similar way to that found for AFP activity in the case of hyperactive insect AFPs.

 Please wait while we load your content...
Please wait while we load your content...