High-valent iron (FeVI, FeV, and FeIV) species in water: characterization and oxidative transformation of estrogenic hormones†
Abstract
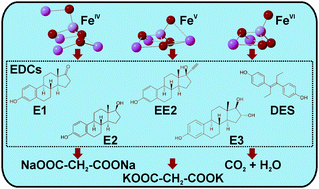
This paper presents solid state synthesis and characterization of tetra-oxy iron(IV) and iron(V) species in their salt forms (Na4FeO4–FeIV and K3FeO4–FeV). Stability of the synthesized salts, commonly called ferrates, in water was determined by applying the 57Fe Mössbauer spectroscopy technique. Within 2 s in water, FeIV converted into FeIII while FeV transformed into FeVI and FeIII at pH = 8.2. Comparatively, FeVI (bought as K2FeO4) remained stable in aqueous solution during the short time period. The oxidative removal efficiency of the high-valent iron species was then tested against five environmentally important estrogenic hormones (estron (E1), 17-β-estradiol (E2), estriol (E3), 17-α-ethinylestradiol (EE2), and diethylstibestrol (DES)) in effluent water of a wastewater treatment plant. Three dosages of iron species (1, 10, and 100 mg L−1) were applied to the effluent water. An increase in the concentration of dosages enhanced the removal of estrogens. Both FeV and FeVI were effective in degrading estrogens, but FeIV showed limited oxidation capacity to transform estrogens. The oxidized products of the estrogens were analyzed using Raman spectroscopy and high-performance liquid chromatography-mass spectrometry (HPLC-MS) techniques. Results demonstrated the transformation of estrogens into low molecular weight oxygenated compounds such as quinone-like and opened-aromatic ring species. A detailed study on E1 by using excess FeVI showed the mineralization of the parent compound. The results demonstrate great potential of high-valent iron species in the degradation of endocrine disruptor chemicals like estrogens with several superior aspects including fast reactions, complete degradation and/or formation of benign organic species, and environmentally-acceptable iron oxide by-products.

 Please wait while we load your content...
Please wait while we load your content...