Influence of oligo(ethylene oxide) substituents on pyrrolidinium-based ionic liquid properties, Li+ solvation and transport
Abstract
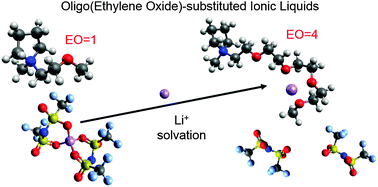
The presence of oligoether functional groups in the cations of ionic liquids has a significant effect on Li+ coordination. In this work, a series of N-alkoxylether-N-methyl pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids were synthesized to investigate the effect of the number of ether units on Li+ coordination and transport. The nature of Li+ coordination was elucidated through the combination of Raman spectroscopy and heteronuclear Overhauser effect NMR spectroscopy. The presence of a simple ether in the cation side chain results in improved physical properties as compared to N-alkyl-N-methyl pyrrolidinium-based ionic liquids, but does not significantly affect Li+ coordination possibly due to steric effects of the pyrrolidinium ring. Increasing the number of ethylene oxide units in the side chain results in the progressive displacement of IL anions in the first Li+ solvation shell by IL cations due to the preferential coordination of Li+ by the ether oxygen atoms. The apparent transference number of the IL cation decreases and that of the IL anion increases with increasing side chain length. Unfortunately, this does not result in an increase in the Li transference. Nonetheless, the results of this study have important implications for electrolyte systems where the desolvation of the metal cation from the IL anions is the limiting factor in the charge transport mechanism.

 Please wait while we load your content...
Please wait while we load your content...