Enhanced photocatalytic activity of a self-stabilized synthetic flavin anchored on a TiO2 surface†
Abstract
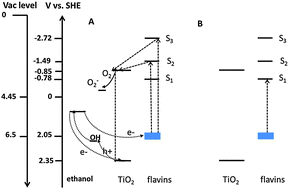
Synthetic flavin molecules were anchored on Degussa P25 titanium dioxide (TiO2). The effect of their presence on the photocatalytic (PC) activity of TiO2 was studied. Under UV light, an increase in the degradation rate of ethanol was observed. This increase was accompanied by stabilization of the anchored flavin against self-degradation. The unprecedented stabilization effect was found also in the absence of a reducing agent such as ethanol. In contrast, under the less energetic visible light, fast degradation of the anchored flavin was observed. These rather surprising observations were attributed to the propensity for charge transport from excited flavin molecules to the semiconductor and to the role that such charge transfer may play in stabilizing the overall assembly. Anchored flavins excited by UV light to their S2, S3 electronic states were able to transfer the excited electrons to the TiO2 phase whereas anchored flavin molecules that were excited by visible light to the S1 state were less likely to transfer the photo-excited electrons and therefore were destabilized. These findings may be relevant not only to anchored flavins in general but to other functionalized photocatalysts, and may open up new vistas in the implementation of sensitizers in PC systems.

 Please wait while we load your content...
Please wait while we load your content...