First principles calculations on oxygen vacant hydrated α-MnO2 for activating water oxidation and its self-healing mechanism†
Abstract
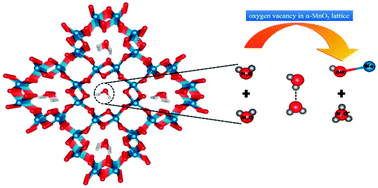
Understanding the mechanism behind water oxidation is the prime requirement for designing better catalysts for electrochemical energy devices. In this work, we demonstrate by employing first principles calculations that an initial step of water oxidation is observed to be associated with the dissociation of water dimers into hydronium and hydroxide ions, in the tunnel of a hydrated α-MnO2 compound with an oxygen vacancy. The former ion is intercalated within the network, while the latter ion occupies the oxygen vacant site and interacts strongly with the Mn atoms. Based on our calculations, the factor responsible for this dissociation of water molecules is observed to be the presence of mixed charge states of Mn atoms in the triangular lattice. Further, the coulombic attraction of a hydronium ion with a water molecule leads to the formation of a Zundel cation in the tunnel, while by dehydrogenating the adsorbed hydroxide ion, the self-healing property of the compound is achieved along with another hydronium ion as a reaction product. These cations can be exchanged with Li+ ions. Thus, the protonic moieties formed in the tunnel of α-MnO2 leads to niche applications in the field of fuel cells and lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...